Cobalt's Secret Electrons—Exposed!
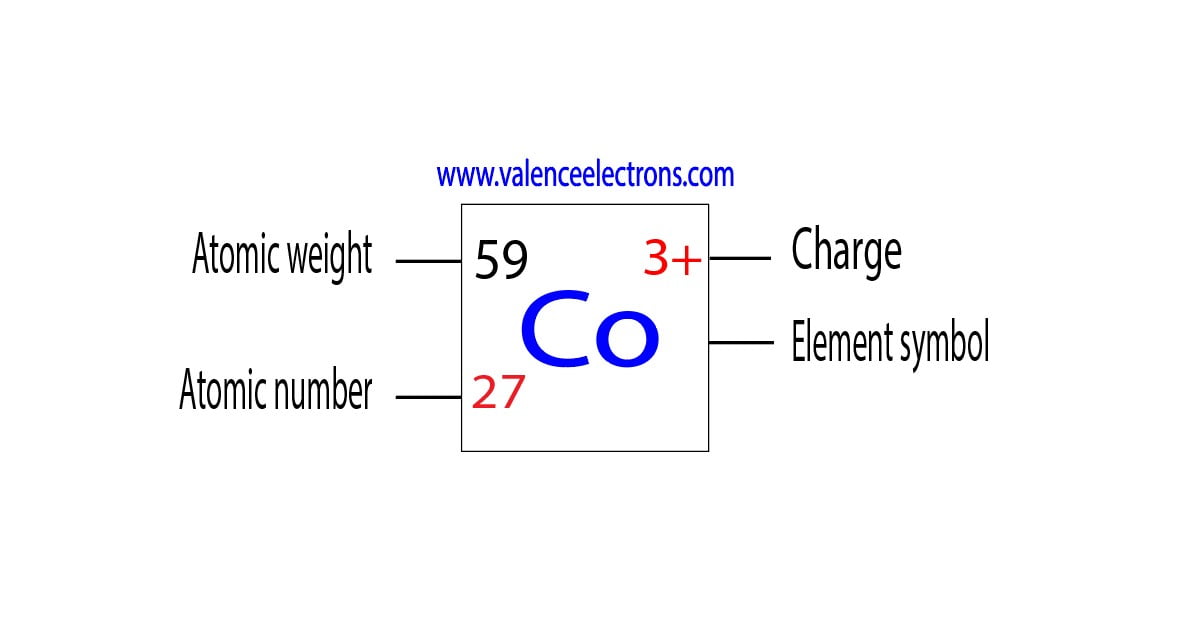
Cobalt, a versatile metal known for its applications in batteries, alloys, and even healthcare, has long fascinated scientists and engineers. But what makes cobalt truly unique? Its electron configuration holds the secret to its remarkable properties. In this post, we’ll uncover the mysteries of cobalt’s electrons, their role in its functionality, and why they matter in industries like technology and energy. Whether you’re a curious learner or a professional in materials science, this guide will shed light on cobalt’s hidden potential. (cobalt properties, electron configuration, materials science)
Understanding Cobalt’s Electron Configuration

Cobalt, with the atomic number 27, has an electron configuration of [Ar] 3d(^7) 4s(^2). This arrangement is key to its magnetic and chemical properties. The 3d electrons are particularly important, as they contribute to cobalt’s ability to form strong bonds and exhibit ferromagnetism. Compared to other transition metals, cobalt’s electron structure makes it a standout choice for high-performance applications. (cobalt electron configuration, transition metals, ferromagnetism)
Why Cobalt’s Electrons Matter in Industry

Cobalt’s electrons play a critical role in its industrial applications. Here’s how:
- Battery Technology: Cobalt’s electrons enable efficient energy storage in lithium-ion batteries, powering smartphones and electric vehicles.
- Alloys: Its electron structure enhances the hardness and corrosion resistance of alloys like superalloys used in aerospace.
- Catalysis: Cobalt acts as a catalyst in chemical reactions, thanks to its reactive electron shell.
These properties make cobalt indispensable in modern technology. (cobalt in batteries, cobalt alloys, cobalt catalysis)
How to Harness Cobalt’s Electron Potential
For businesses and researchers, understanding cobalt’s electrons opens doors to innovation. Here’s a checklist to get started:
- Study cobalt’s electron behavior under different conditions.
- Explore cobalt-based materials for specific applications.
- Invest in sustainable cobalt sourcing to meet industry demands.
📌 Note: Cobalt’s electron configuration is sensitive to temperature and pressure, so precise control is essential in experimentation.
(cobalt innovation, sustainable sourcing, electron behavior)
Cobalt’s Future—Driven by Its Electrons
As industries evolve, cobalt’s electrons will remain at the forefront of advancements. Emerging technologies like solid-state batteries and green energy solutions rely heavily on cobalt’s unique properties. By unlocking its electron secrets, we pave the way for a more sustainable and efficient future. (cobalt future, solid-state batteries, green energy)
Cobalt’s electrons are more than just a scientific curiosity—they’re the foundation of its versatility and importance in modern industries. From powering devices to strengthening materials, cobalt’s electron configuration continues to inspire innovation. By understanding and harnessing this potential, we can drive progress in technology and sustainability. (cobalt applications, technological advancements, sustainability)
What makes cobalt’s electrons unique?
+
Cobalt’s 3d electrons give it unique magnetic and chemical properties, making it ideal for batteries, alloys, and catalysis.
How is cobalt used in batteries?
+
Cobalt enhances the stability and energy density of lithium-ion batteries, making them more efficient and durable.
Why is sustainable cobalt sourcing important?
+
Sustainable sourcing ensures ethical mining practices and meets the growing demand for cobalt in technology.
