T-Shaped Electron Geometry—Science's Dark Secret!
Unveiling the Mystery: T-Shaped Electron Geometry Explained
The world of chemistry is full of fascinating concepts, and one that often sparks curiosity is T-shaped electron geometry. This unique arrangement of electrons around a central atom is a bit of a “dark secret” in the sense that it’s less common than other geometries, but it plays a crucial role in understanding molecular structures and their properties.
What is T-Shaped Electron Geometry?
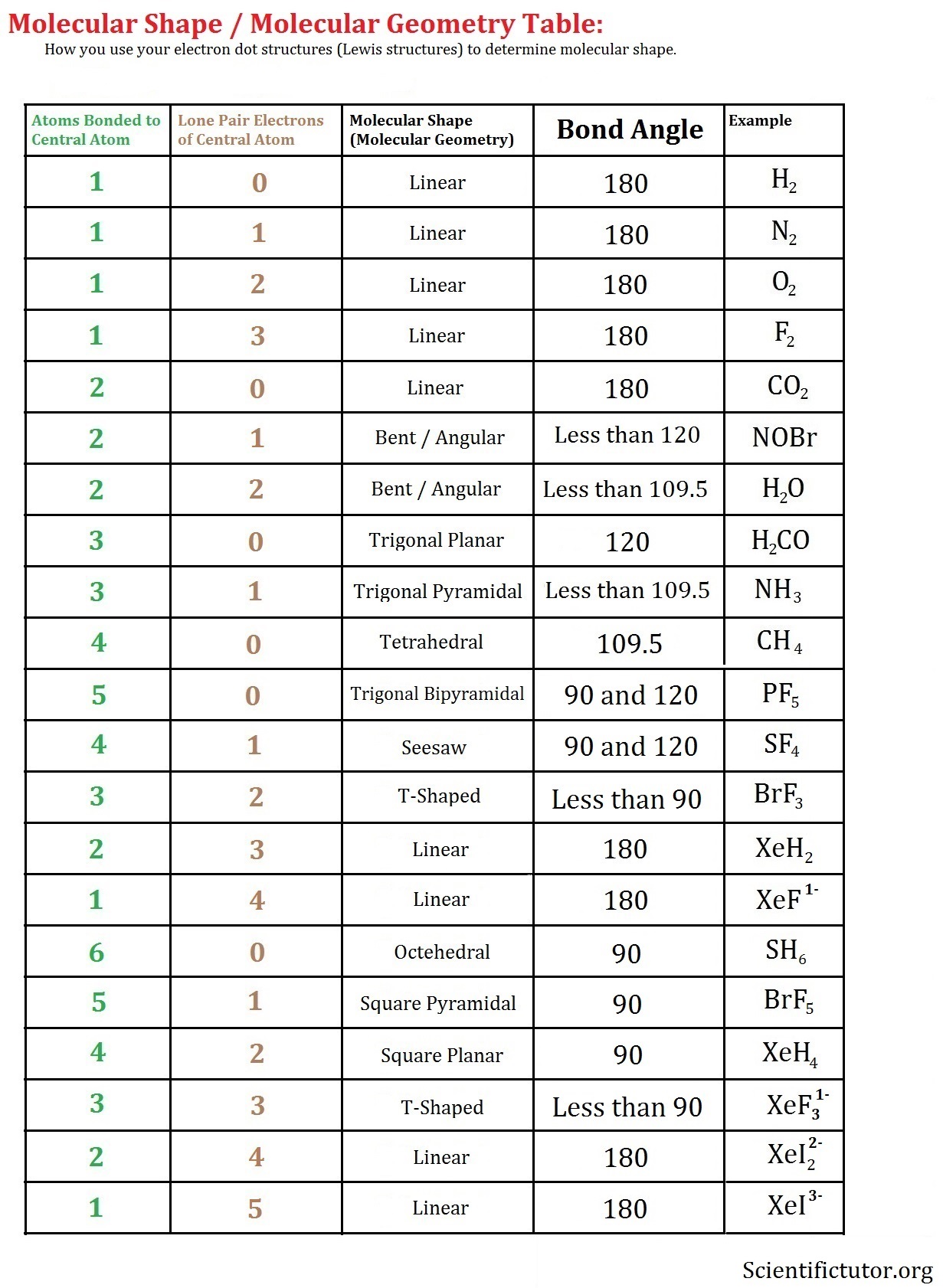
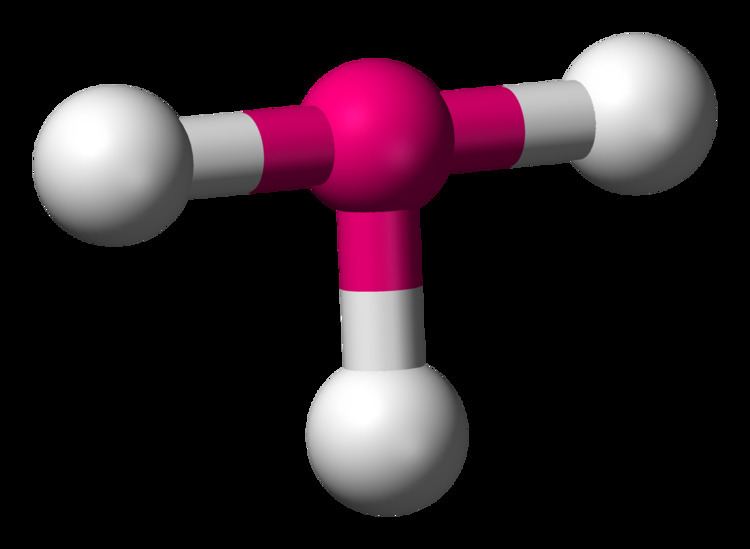
T-shaped geometry occurs when a central atom is surrounded by three regions of electron density, with two bonding pairs and one lone pair. The lone pair repels the bonding pairs more strongly, causing the bonded atoms to form a “T” shape. This geometry is often seen in compounds like ClF3 (Chlorine Trifluoride).
Key Characteristics of T-Shaped Geometry
- Electron Arrangement: 3 regions of electron density (2 bonding pairs, 1 lone pair).
- Bond Angles: Approximately 90° between the bonded atoms, with the lone pair causing distortion.
- Examples: Molecules like ClF3 and BrF3 exhibit T-shaped geometry.
📌 Note: T-shaped geometry is a result of the VSEPR theory, which predicts molecular shapes based on electron pair repulsion.
Why is T-Shaped Geometry Important?
Understanding T-shaped geometry is essential for predicting molecular polarity, reactivity, and physical properties. For instance, the lone pair in T-shaped molecules often makes them more reactive due to increased electron density in that region.
Applications in Real Life
T-shaped molecules are used in various fields, including:
- Chemical Synthesis: As intermediates in complex reactions.
- Material Science: In the development of new materials with unique properties.
- Environmental Chemistry: Studying the behavior of pollutants like ClF3.
How to Identify T-Shaped Geometry
- Count Electron Pairs: Determine the number of bonding and lone pairs around the central atom.
- Apply VSEPR Theory: Use the theory to predict the shape based on electron pair repulsion.
- Analyze Bond Angles: Look for the characteristic 90° angles between bonded atoms.
✨ Note: Tools like molecular modeling software can help visualize T-shaped geometry in 3D.
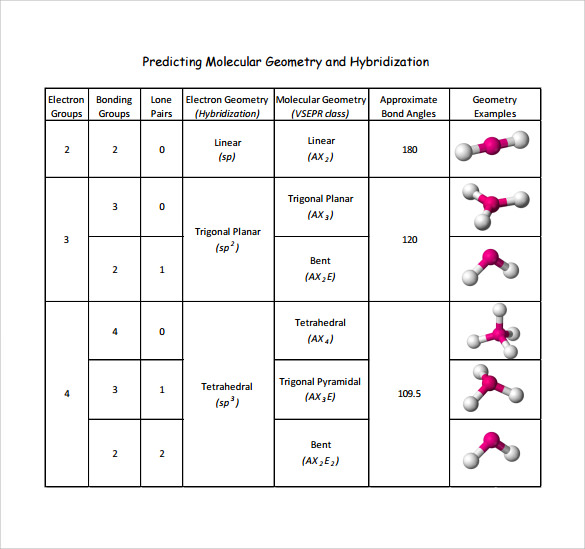
Comparing T-Shaped Geometry with Others
| Geometry | Electron Pairs | Bond Angles | Example |
|---|---|---|---|
| T-Shaped | 3 (2 bonding, 1 lone) | ~90° | ClF3 |
| Trigonal Planar | 3 (all bonding) | 120° | BF3 |
| Linear | 2 (all bonding) | 180° | CO2 |
Final Thoughts
T-shaped electron geometry, though less common, is a fascinating aspect of chemistry that sheds light on the intricate world of molecular structures. By understanding this geometry, scientists can better predict and manipulate the properties of molecules for various applications. Whether you’re a student, researcher, or simply a chemistry enthusiast, grasping this concept opens doors to deeper insights into the molecular world.
What causes T-shaped electron geometry?
+T-shaped geometry is caused by the presence of one lone pair and two bonding pairs around a central atom, with the lone pair repelling the bonding pairs more strongly.
Can T-shaped geometry be polar?
+Yes, T-shaped molecules are often polar due to the asymmetric arrangement of atoms and the presence of a lone pair, which creates an uneven distribution of charge.
What are common examples of T-shaped molecules?
+Common examples include ClF3 (Chlorine Trifluoride) and BrF3 (Bromine Trifluoride).
electron geometry, molecular shape, VSEPR theory, chemical bonding, polar molecules,